By: Cynthia Macdonald
30 Apr, 2019

Dangerous and drug resistant, C. auris thrives almost everywhere
For the past 10 years, Leah Cowen has been watching a deadly pathogen make its way across the world. It is drug-resistant, difficult to diagnose, lives easily on surfaces and targets people with weakened immune systems.
The pathogen is a fungus called Candida auris. As co-director (with Duke University professor Joseph Heitman) of the new CIFAR program Fungal Kingdom: Threats and Opportunities, Cowen is an expert in the biology and evolution of fungal pathogens. These kill more than 1.5 million people per year, and one of the program’s key missions is to address the world’s urgent need for new drug design in the area.
C. auris is neither a bacterium nor a virus, but a form of yeast, which is part of the fungal kingdom.
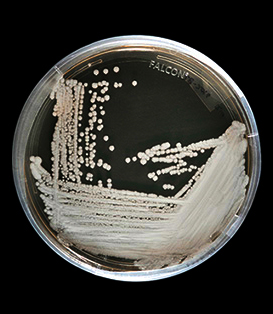
A strain of C. auris cultured in a petri dish
“One problem is that fungi are eukaryotes, which means they’re much more similar to their human hosts than bacteria are,” says Cowen, who is also a professor and chair of molecular genetics at the University of Toronto’s Faculty of Medicine. “Because they share a lot of core essential processes with humans, it’s hard to kill them without producing toxic effects in the patient as well.”
Consequently, very few medications exist to treat fungal illness. “We only have three major classes of antifungals, compared to a dozen classes of antibiotics. And there are some strains that are resistant to everything we have.”
Like bacteria, yeasts normally live on and in us. A common type is Candida albicans, which can cause oral thrush or vaginal infections when spurred out of control (say, by antibiotics taken to combat a bacterial infection.) But though it too is gaining resistance, C. albicans remains highly treatable.
Because they share a lot of core essential processes with humans, it’s hard to kill them without producing toxic effects in the patient as well.”
It also thrives best in warm bodily spaces, whereas C. auris thrives almost everywhere.
“One of the biggest issues is that C. auris seems to survive incredibly well,” says Cowen. “That’s a real problem in hospitals, because it can contaminate all kinds of surfaces.”
C. auris was first identified in 2009, in the ear of a 70-year-old woman in Japan (“auris” is Latin for ear.) Nobody knows where it first started, but one theory is that its resistance may have originated with the overuse of azoles, antifungal agents used in agriculture. Azoles are also the most popular class of drugs used to treat human infections.
The pathogen has been identified in 19 Canadian patients so far. People most seriously affected have already been immunocompromised – whether by cancer, an organ transplant or some other cause. But, as Cowen says, hospitals are full of such people. “And if you have to shut down the intensive care unit of a hospital, that is a really big deal.” Recently, one British hospital was forced to do just that.
C. auris is a reminder that fungi can clearly be deadly, not only to humans but plants and animals. A fifth of the world’s crops die in the field due to fungal diseases, with another tenth being ruined in storage. And a disease known as chitrydiomycosis has led to the extinction of 90 amphibian species in the last 50 years.
Cowen believes that the world is finally taking notice of how dangerous fungi can be. “The tide is turning now,” she says. “This is a crucial moment.”
On the other hand, she points out that fungi can also be beneficial. In fact, some of history’s most helpful medicines – from penicillin to statins – are made by them.
“They have all kinds of impacts, and that’s why we framed our CIFAR program in terms of both threats and opportunities. We want to articulate very specifically that fungi present pretty major threats; but also a tremendous opportunity that we can exploit, to try and solve other problems.”